-
 DHCP
DHCP
-
 Scheelite
Scheelite
-
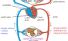 Artery
Artery
-
 Sour cherry
Sour cherry
-
 Differentiation
Differentiation
-
 CAD
CAD
-
 Carbene
Carbene
-
 Melanoma
Melanoma
-
 Heliocentric
Heliocentric
-
 Wafer
Wafer
-
 Magnetic permeability
Magnetic permeability
-
 Corrosion
Corrosion
-
 Position isomerism
Position isomerism
-
 Eclipse of the Sun
Eclipse of the Sun
-
 Tannification
Tannification
-
 Flight feathers
Flight feathers
-
 EJP
EJP
-
 Autumn equinox
Autumn equinox
-
 Stratum
Stratum
-
 Cryogenic
Cryogenic
-
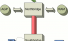 Chipset
Chipset
-
 Okra
Okra
-
 Pixel
Pixel
-
 Diagnosis
Diagnosis
-
 Plasmonics
Plasmonics
-
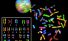 Karyotype
Karyotype
-
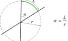 Radian
Radian
-
 Guanosine
Guanosine
-
 London force
London force
-
 BCD galaxy
BCD galaxy
Tungsten
Tungsten is an element (symbol W), of atomic number 74, atomic mass 183.85, belonging to the third series of transition elements. A grey metal of density 19.3 g cm-3, melting point 3410°C (the highest of any metal) and boiling point 5660°C.
Its commonest oxidation state is 6.
Its ores are tungstates such as wolframite, scheelite, stolzite (PbWO4).
Tungsten is obtained by reduction of its oxide, which itself is produced by prior transformation of the ore (fusion in caustic soda).
It is refractory and is used to make arc furnace electrodes and incandescent lamp filaments. Tungsten is used in alloys, especially steels.
Tungsten alloys have been used since the end of the 19th century. Tungsten filaments were introduced by Auer and Coolidge. The evaporation of tungsten, which plays an important role in halogen lamps, was studied by Langmuir. Tungsten carbide tools go back to the 1920s.
Latest
Fill out my online form.



