-
 IMAP
IMAP
-
 Thermocline
Thermocline
-
 Wall lining
Wall lining
-
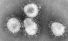 SARS-CoV
SARS-CoV
-
 DAB
DAB
-
 Endoprosthesis
Endoprosthesis
-
 Mucosa
Mucosa
-
 Switch
Switch
-
 Frequency band
Frequency band
-
 Marburg fever
Marburg fever
-
 Gossan
Gossan
-
 Quinolone
Quinolone
-
 Mean motion
Mean motion
-
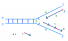 Okazaki fragment
Okazaki fragment
-
 Blog
Blog
-
 Pyrolysis
Pyrolysis
-
 Iapetus
Iapetus
-
 Crystal system
Crystal system
-
 CloudSat
CloudSat
-
 Intercalating
Intercalating
-
 Solenodon
Solenodon
-
 Stencil
Stencil
-
 Basiphilous
Basiphilous
-
 DSL
DSL
-
 Magnetic inversion
Magnetic inversion
-
 Anomalistic period
Anomalistic period
-
 Keratin
Keratin
-
 Mesomerism
Mesomerism
-
 Time
Time
-
 Tungstate
Tungstate
Thermal energy
Thermal energy is the kinetic energy caused by the motion of atoms or molecules in a body. It can be measured in joules (the standard unit of energy), in watt-hours (more commonly used in industry) or in electron-volts (a unit used in physics). The exchange of thermal energy between two bodies is called thermal transfer.
Thermal energy transfer
Heat is a thermal transfer. The temperature measures the mean kinetic energy of the atoms or molecules and shows the capacity of a body to exchange heat. When the atoms or molecules are motionless the temperature reaches an absolute minimum, equal to -273.15°C (degrees Celsius). This is referred to as "absolute zero" and is the origin of temperature measurement in kelvins, K.
When two bodies exchange heat their respective temperatures tend to become equal. When transfer ceases there is thermal equilibrium. Thermal energy transfers occur in three ways: conduction, convection and radiation:
- Conduction is thermal transfer by contact (e.g. two solids, one against the other, or a solid in contact with a motionless fluid);
- convection is thermal transfer between two bodies, at least one of which is a mobile fluid, such as the wind. Because of the motion, thermal equilibrium is difficult to attain. For example, when a hot body is exposed to a cold wind, the body heats the air around it but this layer of heated air is immediately replaced by cold air. Convection is an efficient heat transfer method. It is used in domestic convection heaters improperly called radiators;
- radiation is the emission of electromagnetic waves (black body radiation) of which the frequency spectrum depends on the temperature. A body at a few dozen degrees Celsius, for example, emits in the infrared.
 A body above absolute zero emits electromagnetic radiation. It loses thermal energy through radiation. Here, an engineer at the Goddard centre has just put his hand into his lab coat pocket. A photographer has triggered the shutter of his Qwip camera (NASA) of which the sensor is sensitive to frequencies in the infrared region. © NASA
A body above absolute zero emits electromagnetic radiation. It loses thermal energy through radiation. Here, an engineer at the Goddard centre has just put his hand into his lab coat pocket. A photographer has triggered the shutter of his Qwip camera (NASA) of which the sensor is sensitive to frequencies in the infrared region. © NASA
Latest
Fill out my online form.



