-
 Vector
Vector
-
 Shallot
Shallot
-
 Internal fertilisation
Internal fertilisation
-
 Cladode
Cladode
-
 Maser
Maser
-
 Galileo
Galileo
-
 Baikonur
Baikonur
-
 TPA
TPA
-
 Diffuse pollution
Diffuse pollution
-
 Pandiculation
Pandiculation
-
 Restriction enzymes
Restriction enzymes
-
 POP3
POP3
-
 Juvenile
Juvenile
-
 Condensation
Condensation
-
 CD-WORM
CD-WORM
-
 Moisture regime
Moisture regime
-
 Hawking radiation
Hawking radiation
-
 Deficiency
Deficiency
-
 Plankton
Plankton
-
 Ruderal
Ruderal
-
 Cytopuncture
Cytopuncture
-
 M67
M67
-
 PlayStation 3
PlayStation 3
-
 Multimedia
Multimedia
-
 Fibroplasia
Fibroplasia
-
 Oil spill
Oil spill
-
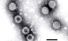 Rotavirus
Rotavirus
-
 Antigorite
Antigorite
-
 UAC
UAC
-
 Achromatopsia
Achromatopsia
Optical activity
Optical activity is a property of certain molecules in solution to cause the rotation of the polarisation plane of polarised light passing through the solution.
This rotatory power is a typical property of chemical compounds that have an asymmetrical structure (cf. chirality).
Amongst these so-called optically active compounds, dextrorotatory compounds divert the polarisation plane clockwise and levorotatory compounds, anticlockwise. Enantiomers, compounds with the same formula of which the structures are symmetrical to each other relative to a plane, have opposite rotatory powers.
Optical activity is studied using polarisation analysers. The measurement of rotatory power is the basis of a physical-chemical analysis method, polarimetry.
The notation + for dextrorotatory and - for levorotatory compounds avoids the confusion that may come about from the use of d and l notations. This confusion may arise because there is no predictable relationship between the relative configurations of asymmetric carbon atoms, noted L and D, and the sign of its rotation.
History of Science The optical activity of chiral substances was observed and studied by Arago and Biot in 1811. Later, in 1848, Pasteur noticed that a chemical reaction usually yielded racemic mixtures because no orientation of asymmetric molecules is favoured. In contrast, in living things one or other configuration is often preferred. The discovery of the link between these optical properties and molecular structure is the basis of stereochemistry which began with the work of Le Bel and van't Hoff. The study of the rotatory power of reaction media goes back to the observation by Walden of the inversion of polarisation that occurs during certain addition reactions.
Latest
Fill out my online form.



