-
 Maar
Maar
-
 Agglutinogen
Agglutinogen
-
 GNSS
GNSS
-
 Isaac Newton
Isaac Newton
-
 Surfactant
Surfactant
-
 Solar power station
Solar power station
-
 Tidal
Tidal
-
 Magnaporthe grisea
Magnaporthe grisea
-
 Crystal face
Crystal face
-
 Brightening
Brightening
-
 Offspring
Offspring
-
 Osteoporosis
Osteoporosis
-
 Mab
Mab
-
 Pallasite
Pallasite
-
 Fly
Fly
-
 Hazardous waste
Hazardous waste
-
 mRNA
mRNA
-
 The theory of punctuated equilibrium
The theory of punctuated equilibrium
-
 Dysaesthesia
Dysaesthesia
-
 CCIR
CCIR
-
 Alar
Alar
-
 Prehensile
Prehensile
-
 Electronic purse
Electronic purse
-
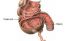 Caecum
Caecum
-
 Gamete
Gamete
-
 RGB
RGB
-
 European corn borer
European corn borer
-
 Verification
Verification
-
 Tricyclic
Tricyclic
-
 Ramsar Convention
Ramsar Convention
Electrolysis
Electrolysis is the chemical decomposition of certain substances by the passage of an electric current.
Electrolysis is carried out in a vessel containing an ELECTROLYTE in which two electrodes are immersed and connected to the terminals of a direct current generator.
The ANODE is the electrode connected to the positive terminal of the generator and the CATHODE is connected to the negative terminal.
This arrangement is an electrolysis cell.
During electrolysis:
· at the ANODE an OXIDATION reaction takes place: hungry for electrons, it behaves as an oxidiser;
· at the CATHODE a REDUCTION reaction takes place: as a source of electrons, it behaves as a reducer.
Electrolysis has many industrial applications such as chromium plating, gold plating, electroplating, chlorine production and the production of sodium hydroxide from sea salt.
As an example, the chromium plating of steel is carried out as follows:
· the metal part used as the CATHODE is immersed with a chromium ANODE in a solution (the ELECTROLYTE) containing chromium ions (Cr3+);
· the generator between the anode and the cathode delivers a direct current;
· the metal part attracts electrons and chromium ions, which adhere to its surface.
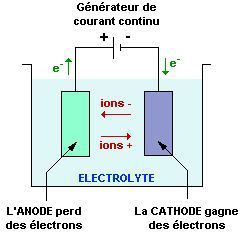
Electrolysis
Latest
Fill out my online form.



