-
 Gasification
Gasification
-
 Technical assessment
Technical assessment
-
 Volcanism
Volcanism
-
 MIME
MIME
-
 Deep Space Network
Deep Space Network
-
 Microgravity
Microgravity
-
 Blandford-Znajek mechanism
Blandford-Znajek mechanism
-
 IETF
IETF
-
 Proof of security
Proof of security
-
 Porphyry copper
Porphyry copper
-
 Bone marrow donor
Bone marrow donor
-
 Phobos-Grunt
Phobos-Grunt
-
 Androgen
Androgen
-
 Aromatic
Aromatic
-
 Conception
Conception
-
 Peristalsis
Peristalsis
-
 Stereoisomer
Stereoisomer
-
 Additional energy
Additional energy
-
 Albinos
Albinos
-
 Automaton
Automaton
-
 WAP
WAP
-
 UV water steriliser
UV water steriliser
-
 NGN
NGN
-
 Pit
Pit
-
 Transcription
Transcription
-
 Conformator
Conformator
-
 Newton
Newton
-
 Granular
Granular
-
 Leukocyte
Leukocyte
-
 Skarn
Skarn
Antibodies
An antibody is a biological molecule involved in immunity.
Structure of antibodies
An antibody is a protein complex. Whilst each organism with an immune system codes for billions of different antibodies, they all have the same overall features. They are glycoproteins belonging to the immunoglobulins family, formed from two identical heavy chains (H for heavy) and two identical light chains (L for light). They are often Y-shaped, with the two heavy chains being linked to each other by a disulphide bridge in the stem of the Y. The two light chains are bound to the heavy chains in the arms of the Y, and also by disulphide bridges.
Antibodies contain constant domains (identical in all antibodies in the same body) and variable domains (which enable recognition of foreign bodies) located at the end of the Y arms. The variable domains form the paratopes of the antibodies.
Function of an antibody
The role of the antibody is to recognise a foreign antigen in order to neutralise it. They achieve this because of their highly specific paratope that only recognises a very specific part of the antigen: the epitope. Once an antibody recognises an epitope, the B lymphocyte, which codes for the specific antibody, multiplies and matures in order to be able to produce large amounts of the same useful antibodies.
There are five different types of antibodies: IgG, IgA, IgM, IgE and IgD (in order of their respective amounts present in the body). They have different roles.
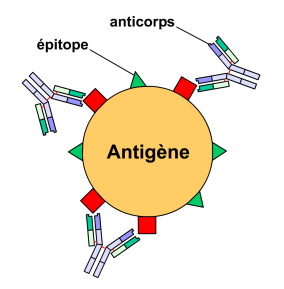
The paratopes of antibodies recognise the epitopes of antigens. © Yohan, Wikimedia, licence GFDL GNU 1.2
Latest
Fill out my online form.



