-
 Chelicerata
Chelicerata
-
 DSL
DSL
-
 Electronic data interchange
Electronic data interchange
-
 Mesohaline water
Mesohaline water
-
 Heliography
Heliography
-
 SACD
SACD
-
 Influenza
Influenza
-
 Main cryogenic stage
Main cryogenic stage
-
 Telescope
Telescope
-
 Declination
Declination
-
 Neoplastic
Neoplastic
-
 Saturn 5
Saturn 5
-
 Anti-diarrhoeal agent
Anti-diarrhoeal agent
-
 XOR
XOR
-
 Marble
Marble
-
 Chromium
Chromium
-
 Isomer
Isomer
-
 Firmicute
Firmicute
-
 Orion nebula
Orion nebula
-
 Chrysalis
Chrysalis
-
 Geostationary transfer orbit
Geostationary transfer orbit
-
 Anti-angiogenic
Anti-angiogenic
-
 Booster
Booster
-
 Biological corridor
Biological corridor
-
 Covolume
Covolume
-
 Common beech
Common beech
-
 Metastatic
Metastatic
-
 Acne
Acne
-
 Insulation
Insulation
-
 CNES
CNES
Graphite
Graphite is a friable mineral which has been used for centuries for writing (Indian ink, "lead" pencil).
The structure of graphite is a stacking of planes, each being made of a regular honeycomb pattern of hexagons.
Each carbon atom is bound to three neighbouring atoms in the hexagon plane by bonds at an angle of 120° from each other. These plane bonds are strong and have a distance of 0.142 nm between atoms.
On the other hand the atoms are weakly bound to the atoms of neighbouring planes with a distance of 0.34 nm between planes.
This structure has a density three times lower than that of diamond and makes graphite a very anisotropic, virtually 2-dimensional solid because the weakly bound planes slide easily over each other.
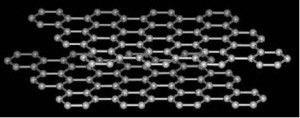
The structure of graphite, from the Greek "graphein" = to write ("lead" pencil, Indian ink).
Latest
Fill out my online form.



