-
 Salt
Salt
-
 Vermiculture
Vermiculture
-
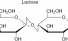 Lactose
Lactose
-
 EER
EER
-
 In vitro
In vitro
-
 SRS
SRS
-
 Flat truss
Flat truss
-
 VSC, PVC
VSC, PVC
-
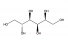 Sorbitol
Sorbitol
-
 Gaugino
Gaugino
-
 Biogas
Biogas
-
 M16
M16
-
 Drought
Drought
-
 Bluetongue disease
Bluetongue disease
-
 Miscible
Miscible
-
 Charadriiformes
Charadriiformes
-
 Mithridatism
Mithridatism
-
 Pelecaniformes
Pelecaniformes
-
 Chandrasekhar limit
Chandrasekhar limit
-
 Anisotropic
Anisotropic
-
 The theory of punctuated equilibrium
The theory of punctuated equilibrium
-
 Petrified
Petrified
-
 Constellation of Gemini
Constellation of Gemini
-
 Systematic
Systematic
-
 Stoma
Stoma
-
 Fluorescence microscope
Fluorescence microscope
-
 ANSI
ANSI
-
 Annealed copper
Annealed copper
-
 IPSec
IPSec
-
 Legionellosis
Legionellosis
Carbon
Carbon is a non-metallic chemical element (symbol C), of atomic number 6, atomic mass 12.01, belonging to group 14 in the periodic classification, and of valency 4.
The element has a melting point of 3550°C and a boiling point of 4827°C; it exists in the solid state in three crystalline forms: graphite, diamond and fullerenes.
Graphite, with the stacking of weakly inter-linked planes of carbon hexagons, is a good electrical conductor; diamond, a crystal with cubic symmetry in which each carbon atom is bound to four atoms at the vertices of a regular tetrahedron, is a good electrical insulator and a good conductor of heat; fullerenes are closed molecules (spheres, cylinders) formed from one or more sheets with a graphite structure with a fraction of the hexagons replaced by pentagons to accommodate the curvature.
There are also amorphous forms such as carbon black.
Carbon is a fuel and a reducing agent; it exists in the natural state, combined in hydrocarbons, in the form of oxides such as carbon dioxide, in carbonates (particularly calcium, as limestone and chalk), and in all organic molecules such as fats, carbohydrates (glucides or sugars) etc.
Latest
Fill out my online form.



