-
 Choroid
Choroid
-
 IPTV
IPTV
-
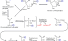 Glycolysis
Glycolysis
-
 Sleet
Sleet
-
 Sex chromosome
Sex chromosome
-
 UTC
UTC
-
 Galactan
Galactan
-
 SEPP
SEPP
-
 Nematode
Nematode
-
 Public domain software
Public domain software
-
 Oncogenesis
Oncogenesis
-
 Caffeine
Caffeine
-
 Clays (minerals)
Clays (minerals)
-
 PlayStation 3
PlayStation 3
-
 Sycamore maple
Sycamore maple
-
 Basitonic
Basitonic
-
 HDCP
HDCP
-
 Adenoma
Adenoma
-
 Scrolling
Scrolling
-
 Phytophage
Phytophage
-
 Analgesic
Analgesic
-
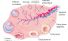 Ovulation
Ovulation
-
 Reduction
Reduction
-
 Fibroblasts
Fibroblasts
-
 Pistil
Pistil
-
 Cytochrome
Cytochrome
-
 M 95
M 95
-
 VHF
VHF
-
 Plaster
Plaster
-
 Weak opiate analgesic
Weak opiate analgesic
Zeolite
Hydrated alumino-silicate. Zeolites ("boiling stone" in Greek) are formed of crystalline structures in which four atoms of oxygen enclose an atom of silicon or aluminium.
They are formed naturally in alkaline water or in sediments and have the property of swelling when heated. Chemically they hydrate and dehydrate reversibly.
There are 48 known natural zeolites but chemists have created nearly 200 synthetic forms. They are used as filters (they have been called molecular sieves) in water softeners, ion exchange columns, oxygen production systems and in the separation of petroleum products and during the extraction of natural gas. They are also used as catalysts in many types of chemical reaction. Some of them are used in agricultural fertilisers as transporting agents for potassium.
Latest
Fill out my online form.



