-
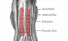 Somite
Somite
-
 Song
Song
-
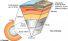 Continental crust
Continental crust
-
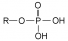 Phosphorylation
Phosphorylation
-
 Primary colour
Primary colour
-
 Windows Vista
Windows Vista
-
 Anti-angiogenic
Anti-angiogenic
-
 Crookes tube
Crookes tube
-
 Heterolysis
Heterolysis
-
 Temperate evergreen forest
Temperate evergreen forest
-
 Alter Eco
Alter Eco
-
 Tarragon
Tarragon
-
 Class III antiarrhythmics
Class III antiarrhythmics
-
 Geomagnetism
Geomagnetism
-
 MBR
MBR
-
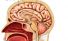 Hypothalamus
Hypothalamus
-
 Amylose
Amylose
-
 Tautomer
Tautomer
-
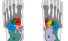 Tarsus
Tarsus
-
 ADEME
ADEME
-
 Dynamo effect
Dynamo effect
-
 Deuterostome
Deuterostome
-
 Solar corona
Solar corona
-
 The theory of punctuated equilibrium
The theory of punctuated equilibrium
-
 Stream bed
Stream bed
-
 Granite
Granite
-
 Acrosome
Acrosome
-
 Sensor
Sensor
-
 Courtship
Courtship
-
 Internal combustion hydrogen engine
Internal combustion hydrogen engine
Van der Waals equation
The Van der Waals equation is the equation of state of real gases named after its discoverer, the Nobel physics prize winner Johannes Diderik Van der Waals. It is an equation used to describe the behaviour of gases in conditions of temperature T and pressure P over a wider range than for ideal gases for a volume V occupied by n moles. It predicts the liquefaction of gases.
It is written in the following form
Experimentally gases do not behave exactly as described by the ideal gas laws. The reasons for this phenomenon are simple:
Firstly, gases are composed of molecules that have a certain volume. Next, there are interactions other than simple elastic shocks between the molecules. As a result, forces of attraction between the molecules will make the pressure of a real gas lower than that of an ideal gas, especially at high pressures.
To take these facts into account van der Waals introduced correction terms into the ideal gas equation PV=nRT. These terms are those involving constants a and b in the above equation and they are determined experimentally.
The covolume b is the volume of the atoms/molecules themselves in one mole of gas and is interpreted as an excluded volume (it is impossible for the volume of gas to be less than b).
The term an2/V2 takes into account the mutual attraction of the atoms/molecules in a real gas. this would later be called the Van der Waals forces between the atoms/molecules.
Although the van der Waals equation is an improvement of the ideal gas equation, it has limitations, and other equations of state better describing liquid and solid phases have since been introduced

Johannes Diderik van der Waals (Credit: 2007 Soylent Communications)
Latest
Fill out my online form.



