-
 Ruptured aneurysm
Ruptured aneurysm
-
 Euphotic zone
Euphotic zone
-
 Quantum computer
Quantum computer
-
 Apollo 11
Apollo 11
-
 CA
CA
-
 Container return scheme
Container return scheme
-
 Drapery
Drapery
-
 Thermal insulator
Thermal insulator
-
 Aplite
Aplite
-
 Invisible Web
Invisible Web
-
 Fibula
Fibula
-
 Homeotic gene
Homeotic gene
-
 Tilia cordata
Tilia cordata
-
 Substitution based encryption
Substitution based encryption
-
 Group velocity
Group velocity
-
 iTunes
iTunes
-
 Goldschmidt classification
Goldschmidt classification
-
 Contracture
Contracture
-
 Monoculture
Monoculture
-
 Space-time diagram
Space-time diagram
-
 Sialidase
Sialidase
-
 Siderite
Siderite
-
 Whitebeam
Whitebeam
-
 Greengage
Greengage
-
 Mascarene petrel
Mascarene petrel
-
 Euhedral
Euhedral
-
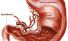 Stomach
Stomach
-
 Cholecystokinin
Cholecystokinin
-
 Absolute magnitude
Absolute magnitude
-
 3G
3G
Salinity
Salinity is the mass of salts contained in 1 kg of seawater. Currently this is obtained by measuring the conductivity and is expressed in psu (practical salinity units), approximately equivalent to 1 mg/g of salts. The salinity of seawater is on average 35 psu, i.e. about 35 g/kg.
Salinity is one of the physical-chemical properties of water.
Salinity measures the concentration of dissolved salts in water (sodium chloride, magnesium chloride, magnesium sulphate etc.) using its electric conductivity.
This salinity has no units, but it is still often expressed in grams of salt per kilogram of water (g/kg), in parts per thousand or in practical salinity units (psu).
Technically, salinity is defined as the mass (in grams) of substances dissolved in one kilogram of seawater when the bromide and iodide ions are replaced by their equivalent in chloride, the carbonates converted into oxides and all of the organic matter is oxidised.
As this value is difficult to obtain, scientists rely on the fact that the proportionality of various salts is constant to define other quantities related to salinity: chlorinity and chlorosity.
As a comparison, fresh water has a salinity from 0 to 0.5 and seawater (euhaline water) has an average salinity of 35.
 Ocean salinity measuring campaign. © Capitaine Robert A. Pawlowski / NOAA
Ocean salinity measuring campaign. © Capitaine Robert A. Pawlowski / NOAA
Latest
Fill out my online form.



