-
 Celsius
Celsius
-
 Acusia
Acusia
-
 Graph theory
Graph theory
-
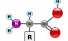 Amino acid
Amino acid
-
 Hubble's law
Hubble's law
-
 Amplexus
Amplexus
-
 Smoltification
Smoltification
-
 Benign
Benign
-
 Ligand
Ligand
-
 Body Mass Index
Body Mass Index
-
 Meridian
Meridian
-
 Neurochip
Neurochip
-
 Composite skeleton
Composite skeleton
-
 Antarctic
Antarctic
-
 Oil producing plant
Oil producing plant
-
 Cryogenian
Cryogenian
-
 UMTS
UMTS
-
 CGB
CGB
-
 Diurnal parallax
Diurnal parallax
-
 Lungfish
Lungfish
-
 PAP
PAP
-
 Newton
Newton
-
 Geophagia
Geophagia
-
 Hydrogel
Hydrogel
-
 Senescence
Senescence
-
 Colitis
Colitis
-
 Noise
Noise
-
 Oven
Oven
-
 Lithospheric
Lithospheric
-
 BOD5
BOD5
Polymerisation
Polymerisation is the process transforming a monomer, or a mixture of monomers, into a polymer.
Polymerisation is a time and temperature dependent chemical reaction which, for thermosets, leads to the irreversible solidification of a matrix or resin.
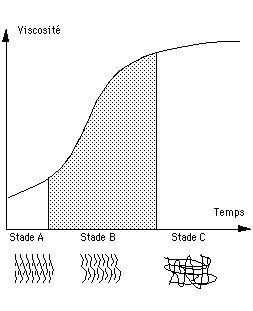
Stages A, B and C define the various states of a thermosetting resin (or system).
At stage "A", the base resin and hardener have not been mixed (or have been mixed but have not yet reacted). They have low viscosity, a low mean molecular mass (the resin containing monomers) and total solubility. If stored correctly, they change little or not at all.
At stage"B", the system (resin + hardener) has started to polymerise (prepolymerisation). This is the gelling stage in the prepregs (the pre-impregnated fibres). This stage is not stable and can change (generally very slowly) to stage "C". To avoid this, the reaction is arrested by cooling.
At stage "B" the system:
• has good mouldability (high viscosity but the material is still fusible);
• lends itself easily to shaping and draping (in prepregs);
• is partially soluble (sometimes still totally soluble);
• has a relatively high mean molecular mass with little residual monomer.
At stage "C", the polymer is cross-linked. It is totally insoluble and infusible.
Polymerisation
Latest
Fill out my online form.



