-
 Chagas Disease
Chagas Disease
-
 Sample
Sample
-
 Gestagen
Gestagen
-
 Complication
Complication
-
 Dehiscence
Dehiscence
-
 Hybrid
Hybrid
-
 Water spouting statue
Water spouting statue
-
 Silurian
Silurian
-
 Data concentrator
Data concentrator
-
 Prime-boost
Prime-boost
-
 Moho
Moho
-
 Blood
Blood
-
 Polydomy
Polydomy
-
 Axial
Axial
-
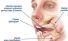 Salivary gland
Salivary gland
-
 Atom
Atom
-
 Agglutinogen
Agglutinogen
-
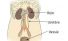 Pyelo-ureteric junction
Pyelo-ureteric junction
-
 Apogee kick motor
Apogee kick motor
-
 Sweet Chestnut
Sweet Chestnut
-
 M38
M38
-
 HQE
HQE
-
 Deficiency
Deficiency
-
 Resistivity
Resistivity
-
 Auditory evoked potential
Auditory evoked potential
-
 Wigner effect
Wigner effect
-
 Brute force
Brute force
-
 Pedicellariae
Pedicellariae
-
 Eccentric anomaly
Eccentric anomaly
-
 Morning-after pill
Morning-after pill
Luminol
Luminol is a chemiluminescent compound (it emits an intense blue light) in the presence of a suitable oxidiser.
Chemistry of luminol
Luminol (or o-aminophthaloyl hydrazide or 3-aminophthalhydrazide) has the molecular formula C8H7N3O2. In the presence of an activator (a hydroxide and hydrogen peroxide) and a catalyst (iron, copper or cyanide), two atoms of nitrogen are detached and are replaced by oxygen atoms and an intense blue light is emitted.
Uses of luminol
It is used in forensic investigations to detect invisible traces of blood because the iron in haemoglobin reacts with luminol.
It is also used in plastified tubes called "glow sticks" that contain an ampoule with the catalyst which, on bending, breaks; the tube then becomes phosphorescent. They are used in entertainments and to indicate danger.
 Luminol mixed with blood and hydrogen peroxide emits blue light. © David Muelheims, Wikimedia, CC by-sa 2.5
Luminol mixed with blood and hydrogen peroxide emits blue light. © David Muelheims, Wikimedia, CC by-sa 2.5
Latest
Fill out my online form.



