-
 Cheilio
Cheilio
-
 Oyashio Current
Oyashio Current
-
 Dialler
Dialler
-
 Chemosynthesis
Chemosynthesis
-
 Claudication
Claudication
-
 Saros
Saros
-
 Blende
Blende
-
 Cross-talk
Cross-talk
-
 Wood pellets
Wood pellets
-
 Biocenosis
Biocenosis
-
 Vinci engine
Vinci engine
-
 Roziere balloon
Roziere balloon
-
 Laser
Laser
-
 Attosecond
Attosecond
-
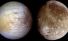 Io
Io
-
 Quartz sand
Quartz sand
-
 Crenocyte
Crenocyte
-
 Pervasive environment
Pervasive environment
-
 Optical disk
Optical disk
-
 Reprotoxic
Reprotoxic
-
 Artificial heart
Artificial heart
-
 Solid solution
Solid solution
-
 Hypothalamic paraventricular nucleus
Hypothalamic paraventricular nucleus
-
 Infusion
Infusion
-
 Arboreal
Arboreal
-
 Equator of a celestial body
Equator of a celestial body
-
 Continental slope
Continental slope
-
 Interferon
Interferon
-
 Android
Android
-
 Isomerism
Isomerism
Lewis formula
The Lewis formula is the structural formula of a molecular species in which all the electrons of the valence shell are represented by points placed in such a way that two points represent an electron pair or a single covalent bond between two atoms.
Note:
1. A double bond is represented by two pairs of points and a triple bond by three pairs.
2. For the sake of simplicity, electron pairs are often represented by a dash.
![]()
Lewis formula for nitrogen.
Latest
Fill out my online form.



