-
 Low temperature boiler
Low temperature boiler
-
 Plateosaurus
Plateosaurus
-
 Meteorite crater
Meteorite crater
-
 Air brake
Air brake
-
 Phenotype
Phenotype
-
 Cave
Cave
-
 Centrosome
Centrosome
-
 Regional metamorphism
Regional metamorphism
-
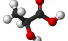 Lactic acid
Lactic acid
-
 ToIP
ToIP
-
 Disseminated deposit
Disseminated deposit
-
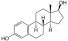 Estrogen
Estrogen
-
 Beta-2 stimulant
Beta-2 stimulant
-
 Verification
Verification
-
 Plug-in
Plug-in
-
 Acoustic crest
Acoustic crest
-
 Cytokine
Cytokine
-
 Secunia
Secunia
-
 Apron
Apron
-
 Dry process
Dry process
-
 Cyanobacteria
Cyanobacteria
-
 Atheroma
Atheroma
-
 Voice synthesis
Voice synthesis
-
 Jerusalem artichoke
Jerusalem artichoke
-
 Heliopause
Heliopause
-
 Okra
Okra
-
 Granivore
Granivore
-
 Pluto
Pluto
-
 Mars Express
Mars Express
-
 DTS
DTS
Latest
Fill out my online form.



