-
 Convection
Convection
-
 Aerocapture
Aerocapture
-
 Relative permittivity
Relative permittivity
-
 S/PDIF
S/PDIF
-
 Ubiquitin
Ubiquitin
-
 Tetanus
Tetanus
-
 Arthritis
Arthritis
-
 FDMA
FDMA
-
 Chemical mediator
Chemical mediator
-
 Mauritius parakeet
Mauritius parakeet
-
 Anaesthesia
Anaesthesia
-
 Exocrine
Exocrine
-
 Turnip
Turnip
-
 Donders rings
Donders rings
-
 Chondrosarcoma
Chondrosarcoma
-
 Maintenance
Maintenance
-
 Laparoscopy
Laparoscopy
-
 Heliosphere
Heliosphere
-
 Coupled reactions
Coupled reactions
-
 Internal combustion hydrogen engine
Internal combustion hydrogen engine
-
 Pandiculation
Pandiculation
-
 Wing tip
Wing tip
-
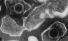 Epstein-Barr virus
Epstein-Barr virus
-
 Dew point
Dew point
-
 Coalescence
Coalescence
-
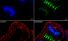 Fluorescence microscope
Fluorescence microscope
-
 Endergonic
Endergonic
-
 Fusiform
Fusiform
-
 European eel
European eel
-
 Formaldehyde
Formaldehyde
Hardness of water
The hardness of water gives the concentration of carbonates (CO32-) and strong bases, in other words its alkalinity.
Different scales are used in different countries; in the UK, English degrees (°e) are used and in the USA, ppm, also called American degrees. 1°e is equivalent to 2.39 mg/L of hydroxide ion (HO-) or 4.21 mg/L of carbonate ion, or 8.56 mg/L of bicarbonate ion (HCO3-).
To take into account the bicarbonate ions (HCO3-), the total alkali strength is used. Total hardness is expressed as the sum of the calcium and magnesium concentrations.
Thealkalinity of water is closely related to its hardness and therefore to its corrosive character and capacity to produce scale in pipes.
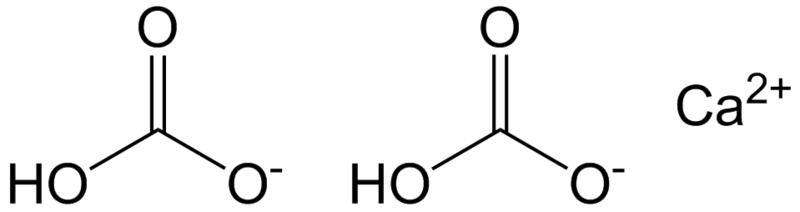
A calcium bicarbonate molecule in solution. Total alkali strength indicates bicarbonate ions. © Epop, Wikimedia public domain
Latest
Fill out my online form.



