-
 Tumour antigen
Tumour antigen
-
 MRI
MRI
-
 Chlamydia
Chlamydia
-
 Passive continental margin
Passive continental margin
-
 Leprosy
Leprosy
-
 Allotropy
Allotropy
-
 Allosteric enzyme
Allosteric enzyme
-
 Small planet
Small planet
-
 Coralloid
Coralloid
-
 Launch campaign
Launch campaign
-
 Amputation
Amputation
-
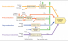 Post-combustion CO2 separation
Post-combustion CO2 separation
-
 Magnetic field
Magnetic field
-
 Svalbard Global Seed Vault
Svalbard Global Seed Vault
-
 Riparian plant
Riparian plant
-
 Chlordecone
Chlordecone
-
 Zebrafish
Zebrafish
-
 GNU
GNU
-
 Polymetallic
Polymetallic
-
 Song
Song
-
 Homoptera
Homoptera
-
 Acini
Acini
-
 Insect
Insect
-
 Platinum
Platinum
-
 CD-V
CD-V
-
 Grapefruit
Grapefruit
-
 Galileo
Galileo
-
 Hotspot
Hotspot
-
 Extinction
Extinction
-
 Irradiation
Irradiation
Empirical formula
An empirical formula is one consisting of atomic symbols giving the simplest possible expression of the stoichiometric composition of a compound that agrees with the results of elementary quantitative analysis.
Note:
1. For example, the molecular formula of all the oses, such as glucose, is CH2O.
2. The expression "molecular formula" takes into account the actual number of each atom in the molecule. For example, the molecular formula of glucose is C6H12O6.
Latest
Fill out my online form.



