-
 Eusocial
Eusocial
-
 Mach number
Mach number
-
 Archaeopteryx
Archaeopteryx
-
 Tombolo
Tombolo
-
 Half-life
Half-life
-
 Phobos-Grunt
Phobos-Grunt
-
 Indehiscent
Indehiscent
-
 VoIP
VoIP
-
 Kinetic energy
Kinetic energy
-
 Steppe
Steppe
-
 Transfusion
Transfusion
-
 Ischaemic
Ischaemic
-
 Lagrangian point
Lagrangian point
-
 Lamellar
Lamellar
-
 Atypical antipsychotic
Atypical antipsychotic
-
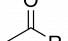 Acetylation
Acetylation
-
 Combe
Combe
-
 Peridotite
Peridotite
-
 Habitable zone
Habitable zone
-
 Patella
Patella
-
 Taxonomy
Taxonomy
-
 Fractal
Fractal
-
 Extinction
Extinction
-
 Dolomite
Dolomite
-
 Small Magellanic cloud
Small Magellanic cloud
-
 Pont Farin
Pont Farin
-
 WAP
WAP
-
 Electroencephalogram
Electroencephalogram
-
 Chrysalis
Chrysalis
-
 Lobed
Lobed
Cross linking
Cross linking is the interconnection of polymer chains by chemical bridges or bonds, for example under the action of radiation, in order to make a network of higher molecular mass with different physical-chemical properties from the initial polymer, e.g. insolubility in solvents;
Cross linking causes a thermosetting resin to go from the paste form to the solid state. Cross linking is a final product of polymerisation and is irreversible.
In thermosetting polymers, cross linking corresponds to the formation of a three-dimensional macromolecular network.
The polymer passes from a paste form (viscoelastic) to a rigid, elastic and infusible solid state. The gel state is the beginning of cross linking. The cross linking mechanism differs according to the resin but only applies to multifunctional molecules.
It can be by:
• thermal or catalytic activation of reactive sites (phenolic resins of the resol type);
• cross linking by the addition of a reactive monomer in the resin (polyester) ;
• reaction with a hardener (epoxides).
The vulcanising of rubber is an example of cross linking.
Latest
Fill out my online form.



