-
 LOX
LOX
-
 Spiral galaxy
Spiral galaxy
-
 Vitamin C
Vitamin C
-
 CSA
CSA
-
 Interactive kiosk
Interactive kiosk
-
 Shared resource radio network
Shared resource radio network
-
 Nadir
Nadir
-
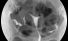 Hysterography
Hysterography
-
 Sweet chestnut
Sweet chestnut
-
 Congruence
Congruence
-
 CD-V
CD-V
-
 Frenulum
Frenulum
-
 Embryo
Embryo
-
 Mangrove
Mangrove
-
 Monomer
Monomer
-
 Orbit
Orbit
-
 Amyotrophy
Amyotrophy
-
 Wind turbine
Wind turbine
-
 Mesohaline water
Mesohaline water
-
 Low temperature boiler
Low temperature boiler
-
 Glucose-6-phosphate
Glucose-6-phosphate
-
 Hydrolysis
Hydrolysis
-
 Antiviral
Antiviral
-
 Rotterdam Convention
Rotterdam Convention
-
 Circuit switching
Circuit switching
-
 Multiple sclerosis
Multiple sclerosis
-
 Canada-France-Hawaii observatory
Canada-France-Hawaii observatory
-
 Dwarf planet
Dwarf planet
-
 Common channel signalling
Common channel signalling
-
 Eruption
Eruption
Coordination number
The coordination number is the number of atoms, molecules or ions directly bonded to a central atom in a molecular species.
Notes:
1. Examples: in methane CH4 the coordination number of carbon est 4; that of cobalt is 6 in the complex cation hexaamminecobalt Co(NH3)63+.
2. The expression is used in a different sense in the geometrical description of ionic crystals; here it is the number of nearest neighbours to the ion in question. Example: in sodium chloride the ions form a regular lattice in which each ion has a coordination number of 6.
Latest
Fill out my online form.



