-
 Geoportail
Geoportail
-
 Conformation
Conformation
-
 Triazine
Triazine
-
 CFM
CFM
-
 Tunnel effect
Tunnel effect
-
 Gas carrier
Gas carrier
-
 Fennel
Fennel
-
 Recombination
Recombination
-
 Ophidian
Ophidian
-
 Iron bloom
Iron bloom
-
 Crenellation
Crenellation
-
 Vacuum permeability
Vacuum permeability
-
 Amniote
Amniote
-
 Vanograph
Vanograph
-
 Mesosphere
Mesosphere
-
 Recovery
Recovery
-
 Voice recognition
Voice recognition
-
 Q fever
Q fever
-
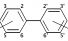 PCB
PCB
-
 Gametangium
Gametangium
-
 Botryoidal
Botryoidal
-
 Clinical trial
Clinical trial
-
 Homotopy
Homotopy
-
 Biogas
Biogas
-
 Deep Impact
Deep Impact
-
 Contrast
Contrast
-
 Telluric pollution
Telluric pollution
-
 Orphan disease
Orphan disease
-
 Ependymoma
Ependymoma
-
 Parkinson's Disease
Parkinson's Disease
Calcium
Calcium is an element (symbol Ca) of atomic number 20, atomic mass 40.08, a solid, soft silvery-white alkaline earth metal, of density 1.55 g/cm3, melting point 845°C, boiling point 1484°C.
Obtained from calcium chloride electrolytically. Calcium is a very strong reducing agent and is used in calciothermy (exothermic reduction) to prepare certain metals such as uranium, plutonium and thorium.
Calcium was isolated in 1808 by Davy by electrolysing calcium carbonate.
It is abundant in the Earth's crust, especially in the form of limestone (calcium carbonate) and sulphates (gypsum).
Calcium is present in water in the form of mineral salts (Ca2+ ion). It is present in relatively large quantities in living matter, especially in blood (the level of calcium in the blood is called calcemia) and is vital to bone growth.
Intracellular calcium concentration is maintained at between 20 and 300 nM by constant calcium pumping. An increase in its concentration triggers a large number of cellular reactions from the release of hormones to cellular contraction, proliferation or apoptosis.
A study of some of the spectral lines of calcium led to the discovery of the red shift.
Latest
Fill out my online form.



