-
 Dolerite
Dolerite
-
 Hackberry
Hackberry
-
 Protocol
Protocol
-
 Americium
Americium
-
 Caprimulgiformes
Caprimulgiformes
-
 Transgenic plant
Transgenic plant
-
 Exocytosis
Exocytosis
-
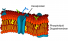 Plasma membrane
Plasma membrane
-
 Ketoacidosis
Ketoacidosis
-
 Daemon
Daemon
-
 Bunsen Burner
Bunsen Burner
-
 Chloracne
Chloracne
-
 Melanism
Melanism
-
 GMO
GMO
-
 Acari
Acari
-
 Echinoderm
Echinoderm
-
 Crystal lattice
Crystal lattice
-
 Bandage
Bandage
-
 MIDI
MIDI
-
 Alkaline
Alkaline
-
 Mesoglea
Mesoglea
-
 Vitamin D
Vitamin D
-
 Cyborg
Cyborg
-
 XOR
XOR
-
 Hot spring
Hot spring
-
 Remote data processing
Remote data processing
-
 Twinned crystal
Twinned crystal
-
 Chronic
Chronic
-
 Melanin
Melanin
-
 RSPB
RSPB
Absolute zero
Just as the speed of light is an unattainable limiting speed for a physical system, there is also a lower unattainable limit for the thermodynamic temperature of a physical system introduced by Kelvin: absolute zero. On the degree Celsius scale, it is exactly -273.15°C, by convention. In classical physics it corresponds to the absence of motion on the microscopic scale for a physical system such as a gas. In reality, due to quantum mechanics and the Heisenberg inequalities, there is a residual state of motion corresponding to the minimum energy for a physical system. At this level of energy the temperature and even the entropy of the system are then zero.
For entropy, this is only rigorously accurate for a perfect crystal, and it is the variations in the entropy of a system that tend to zero with the thermodynamic temperature according to Nernst's theorem. The current lowest temperature attained is of the order of 450 ±80 °K in 2003. Note that some systems, such as those made up of nuclei having spins without interactions show a negative temperature when placed in a magnetic field. Based on the direction in which heat transfer occurs in these systems in thermal contact with another system at a positive temperature , the result is that the negative temperatures are higher than the positive ones!
Latest
Fill out my online form.



