-
 RC4
RC4
-
 CFC
CFC
-
 Homolysis
Homolysis
-
 Granivore
Granivore
-
 Palm
Palm
-
 Omega-6/omega-3 ratio
Omega-6/omega-3 ratio
-
 Mesomerism
Mesomerism
-
 Bone marrow donor
Bone marrow donor
-
 Cranberry
Cranberry
-
 Switched virtual circuit
Switched virtual circuit
-
 Endocrine system
Endocrine system
-
 Hot Jupiter
Hot Jupiter
-
 Enteric fever
Enteric fever
-
 Crystalline aggregate
Crystalline aggregate
-
 Curietherapy
Curietherapy
-
 Cholecystokinin
Cholecystokinin
-
 Lymphocyte
Lymphocyte
-
 Colitis
Colitis
-
 Pasture
Pasture
-
 Metabolic crossover
Metabolic crossover
-
 Crude oil
Crude oil
-
 dMRI
dMRI
-
 Antibiotic
Antibiotic
-
 Hereditary disease
Hereditary disease
-
 Quantum computer
Quantum computer
-
 Gastrulation
Gastrulation
-
 Glabella
Glabella
-
 Radiometer
Radiometer
-
 Vertebral column
Vertebral column
-
 Internal fertilisation
Internal fertilisation
Sumoylation (SUMOylation)
Sumoylation is one of the post-translational modifications commonly seen in proteins.
This involves the covalent binding of a peptide called SUMO to specific sites on the proteins made up of lysine amino acids.
Sumoylation occurs in three stages and involves 2 to 3 catalytic enzymes:
- maturation: cleavage of the C-terminal end of the peptide SUMO;
- activation: creation of a thioester link between SUMO and the binding enzyme;
- conjugation: the activated SUMO is bound to the proteins.
Four types of SUMO proteins have been described until now.
Sumoylation leads to a change in the sumoylated protein: it may enable it to recruit a new partner or change its conformation.
Although related to ubiquitination (which leads to degradation of the target protein) in its biochemical process, sumoylation enables very different functions. These include regulation of the cell cycle, stabilisation of the protein, nuclear-cytoplasmic transport, regulation of transcription, etc.
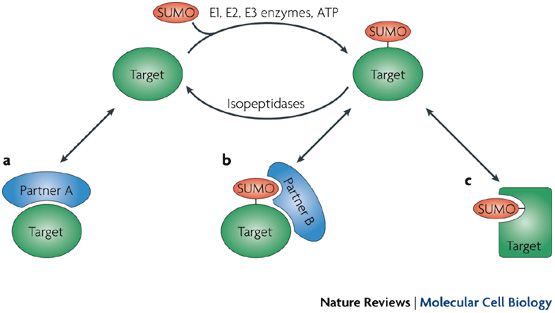 Sumoylation causes a change in the target protein. a) the non-sumoylated protein can bind to the partner A, b) the sumoylated protein combines to a new protein B, but no longer with A, c) the protein changes conformation and no longer binds. © Nature reviews / Molecular Cell Biology
Sumoylation causes a change in the target protein. a) the non-sumoylated protein can bind to the partner A, b) the sumoylated protein combines to a new protein B, but no longer with A, c) the protein changes conformation and no longer binds. © Nature reviews / Molecular Cell Biology
Latest
Fill out my online form.



